Scanning probe microscopy technique could open the door to molecular machines
This week’s Science cover story demonstrates how to use a scanning probe microscope for selective chemical bond formation.
Selective chemistry — the ability to steer reactions at will and to form exactly the chemical bonds you want and no others — is a long-standing quest in chemistry. Our team has been able to achieve this level of selectivity in tip-induced redox reactions using scanning probe microscopy. Our technique consisted in using the tip of a scanning probe microscope to apply voltage pulses to single molecules.
We were able to target specific chemical bonds in those molecules, breaking those bonds and forging new, different ones to switch back and forth at will among three different molecular structures. The molecules in our experiment all consisted of the same atoms,” but differed in the way those atoms were bonded together and arranged in space. This is what chemists call constitutional isomers.
Our This work is fruit of a collaboration of groups at IBM Research Europe – Zurich, CIQUS at the University of Santiago de Compostela, King Abdullah University of Science and Technology (KAUST), and University of Regensburg. It was funded by the ERC Synergy Grant MOLDAM and the EU FET-open project SPRING.findings were published today and featured on the cover of Science .1 Our demonstration of selective and reversible formation of intramolecular covalent bonds is unprecedented. It advances our understanding of chemical reactions and opens a route towards advanced artificial Molecular machines are molecules that perform a task in response to an external stimulus. Naturally occurring, biological macromolecular machines fulfill important functions for life, for example in DNA replication. Artificial molecular machines, on the other hand, are specifically designed and lab synthesized, and sometimes much smaller molecules. They help us improve our understanding of how molecular machines work and might lead to novel unforeseen applications.molecular machines.
This field of research was recognized in 2016 with the Nobel Prize in Chemistry was awarded to Jean-Pierre Sauvage, J. Fraser Stoddart, and Ben L. Feringa for the design and synthesis of molecular machines. A team led by Ben L. Feringa demonstrated2 a molecular nanocar driven on a surface by voltage pulses from a In 1981, IBM scientists Gerd Binnig and Heinrich Rohrer invented the scanning tunneling microscope, which won the Nobel Prize in Physics in 1986.scanning tunneling microscope tip. More complex tasks might become possible in the future if different stimuli trigger different responses of a molecule.
Same atoms, different molecules
In our experiments, we used molecules with the same atomic composition but different arrangement of bonds between their constituent atoms — what chemists call structural or constitutional isomers. Carbon-based compounds are known to exhibit a variety of structural isomers, which is decisive for the complexity in biochemistry and for life in general.
Imagine one could rearrange bonds inside a molecule at will, transforming one structural isomer into various other ones in a controlled manner. In this paper, we describe a system and a method to make exactly that possible — including the control of the direction of the atomic rearrangements by means of an external driving voltage, and without the use of reagents.
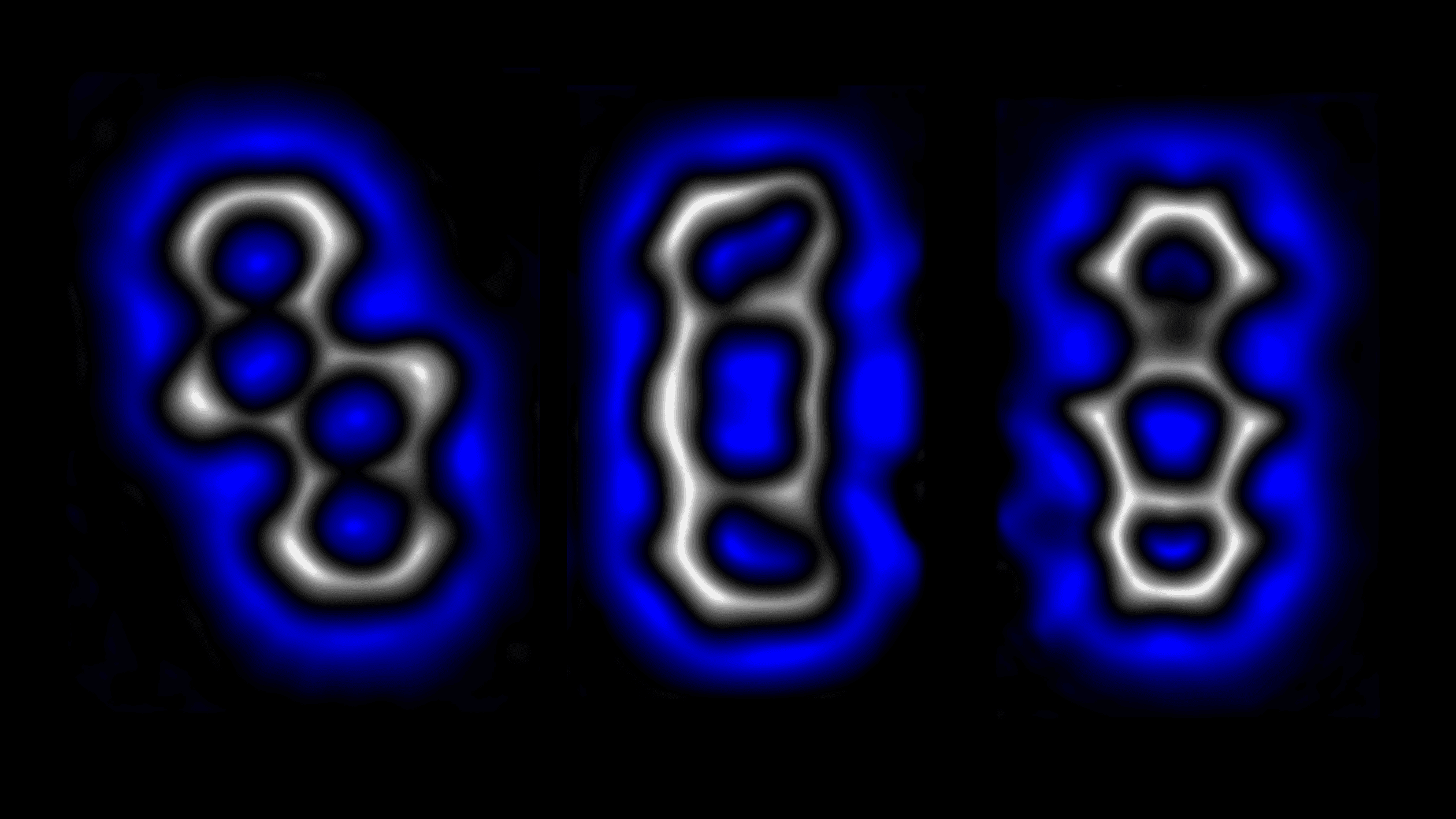
We used a combined scanning tunneling and atomic force microscope working in ultrahigh vacuum and at a temperature of -450.67°F or -268.15°C5 Kelvin. Precursor molecules, synthesized at CiQUS, Santiago de Compostela, were deposited on an ultrathin (two monolayer thick) NaCl film on a copper single crystal. Using the tip of a scanning probe microscope, we first dissociated chlorine masking groups from the precursor, forming a carbon-rich, highly strained molecule.
On this resulting molecule we demonstrated that different internal bonds can be formed and broken at will. Depending on the exact value of voltage pulses applied from the tip of the microscope, the structure of the molecule that features a 10-membered carbon ring in the center can be either converted to a molecule with a 4- and an 8-membered ring, or a structure with two 6-membered rings in its center. The reactions are reversible; we can therefore switch from one molecular strcutre to another repeatedly and in a controlled fashion. Such selective and reversible bond formation could enable molecular machines that perform variable and complex tasks.
We investigated the mechanisms that enabled the selectivity in these reactions and found that an important ingredient for the selectivity is the energy landscape of the molecule in different charge states. Different charge states are addressed by voltage pulses of different values .Additionally, energy is transferred by tunneling electrons. The insights we obtained are useful to better-understand redox reactions and molecular transformations in general that are important in organic synthesis and in nature.
Now that we found this possibility to control bond formation with selectivity, and advanced the understanding in the underlying mechanisms, we will build upon this knowledge. Complex molecular machines and more complex tasks triggered and selected by voltage pulses could become possible.
For example, another path to explore further could be to trigger the reactions not by electrons from a tip of a scanning tunneling microscope, but by transferring electrons between different sites within the molecule. The relatively high energy barriers of the triggered reactions indicate that significant workload could be performed by molecular machines based on selective bond formation.
Notes
- Note 1: This work is fruit of a collaboration of groups at IBM Research Europe – Zurich, CIQUS at the University of Santiago de Compostela, King Abdullah University of Science and Technology (KAUST), and University of Regensburg. It was funded by the ERC Synergy Grant MOLDAM and the EU FET-open project SPRING. ↩︎
- Note 2: Molecular machines are molecules that perform a task in response to an external stimulus. Naturally occurring, biological macromolecular machines fulfill important functions for life, for example in DNA replication. Artificial molecular machines, on the other hand, are specifically designed and lab synthesized, and sometimes much smaller molecules. They help us improve our understanding of how molecular machines work and might lead to novel unforeseen applications. ↩︎
- Note 3: In 1981, IBM scientists Gerd Binnig and Heinrich Rohrer invented the scanning tunneling microscope, which won the Nobel Prize in Physics in 1986. ↩︎
- Note 4: -450.67°F or -268.15°C ↩︎
References
-
Albrecht, F. et al. Selectivity in single-molecule reactions by tip-induced redox chemistry. Science vol. 377 298–301 (2022). ↩
-
Kudernac, T., Ruangsupapichat, N., Parschau, M. et al. Electrically driven directional motion of a four-wheeled molecule on a metal surface. Nature 479, 208–211 (2011). ↩
Related posts
- Deep DiveJuan Bernabé-Moreno
Introducing Thinking-in-Modalities with TerraMind
Technical noteBenedikt Blumenstiel and Johannes JakubikAI-powered satellites will upend how we observe our changing planet
ReleaseMike MurphyHere comes a foundation model for the Sun
ReleaseKim Martineau and Mike Murphy